Welcome to our guide on the Common Technical Document (CTD) quality standards, where we make sense of the rules for pharmaceutical submissions. If you’ve ever felt lost in the maze of regulations, fear not! We’re here to make it simple and straightforward.
Introduction: What’s the Deal with CTD?
Imagine you’re getting ready to submit your medicine to the FDA. Among all the paperwork, you come across the CTD – a way to organize your documents. But where do you start?
Our guide series breaks it down, starting with the Quality section – a crucial part of your application that needs to be spot on.
Why Does CTD Matter?
The CTD saves time and money by giving everyone the same playbook. With clear rules, it’s easier for regulators to understand your medicine and for you to talk to them.
What’s in This Guide?
We’re talking about the Quality part today, but don’t worry, we’ll cover everything else too. Our goal is to help you understand all aspects of the CTD so you can nail your submission.
Tips for Success
We’ll make sure your document is easy to read with clear fonts and plenty of space. Plus, we’ll explain any tricky terms along the way.
What’s Next?
There are five modules to go through, each with its own purpose. Stick with us, and we’ll guide you through each step of the process.
Diagrammatic Representation of the ICH Common Technical Document
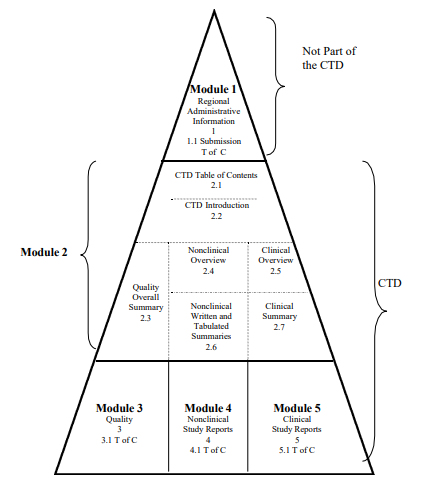
MODULE 1: ADMINISTRATIVE INFORMATION AND PRESCRIBING INFORMATION
- Table of Contents of the Submission Including Module 1 1.2 Documents Specific to Each Region (for example, application forms, prescribing information).
Module 1 of the CTD is region-specific and contains administrative information and prescribing information relevant to the region in which the drug product application is being submitted. The specific requirements for the content of Module 1 can vary depending on the regulatory authority of the region. However, it generally includes:
– Application forms or other documents required by the region.
– The proposed label for the product use in the region.
– Administrative information that may include the proprietary name, non-proprietary name or common name of the drug substance, company name, dosage forms, strengths, route of administration, and proposed indications.
MODULE 2: QUALITY OVERALL SUMMARY
Module 2 of the CTD, known as the Quality Overall Summary (QOS), is a detailed summary that reflects the information contained in Module 3. It is structured to provide a comprehensive overview of the drug substance and drug product, and it should not introduce new data or justifications not already included in Module 3 or other parts of the CTD. The specific requirements for Module 2 include:
1. Introduction: that outlines the proprietary name, non-proprietary name, or common name of the drug substance, company name, dosage forms, strengths, route of administration, and proposed indications.
2. For the drug substance:
– General Information: A summary of the drug substance’s general information.
– Manufacture: A brief description of the manufacturing process, controls, and a flow diagram.
– Characterization: A summary of the evidence of structure and isomerism, and for biotech products, a description of the product and related substances.
– Control of Drug Substance: A summary of the justification of the specifications and analytical procedures.
– Reference Standards or Materials: Information on the reference standards or materials used.
– Container Closure System: A description and discussion of the container closure system.
– Stability: A summary of stability studies, proposed storage conditions, and shelf life.
3. For the drug product:
– Description and Composition: Information on the drug product’s description and composition.
– Pharmaceutical Development: A discussion of the pharmaceutical development data.
– Manufacture: A brief description of the manufacturing process and controls.
– Control of Excipients: A summary of the quality of excipients.
– Control of Drug Product: A summary of the justification of the specifications and analytical procedures.
– Reference Standards or Materials: Information on the reference standards or materials used.
– Container Closure System: A description and discussion of the container closure system.
– Stability: A summary of stability studies and analysis of data.
4. Appendices
– Facilities and Equipment: Information on the facilities and equipment used.
– Adventitious Agents Safety Evaluation: An evaluation of the safety of adventitious agents.
– Novel Excipients: Information on novel excipients used in the drug product.
5. Regional Information: Specific information relevant to the region where the drug product application is being submitted.
The QOS should not exceed 40 pages of text, excluding tables and figures, for most products, and 80 pages for biotech products or those manufactured using more complex processes. It should include cross-referencing to volume and page number in other modules, particularly when discussing key issues that integrate information from different sections of the Quality module and supporting information from other modules.
MODULE 3: QUALITY
Module 3 of the CTD, which is the Quality section, is comprehensive and detailed. It is divided into two main parts: 3.2.S for the Drug Substance and 3.2.P for the Drug Product. Here are the specific requirements for each:
For the Drug Substance (3.2.S):
– General Information: Nomenclature, structure, and general properties of the drug substance.
– Manufacture: Information on manufacturers, a description of the manufacturing process and process controls, control of materials, controls of critical steps and intermediates, process validation and/or evaluation, and manufacturing process development.
– Characterization: Elucidation of structure and other characteristics, impurities.
– Control of Drug Substance: Specifications, analytical procedures, validation of analytical procedures, batch analyses, and justification of specification.
– Reference Standards or Materials: Information on the reference standards or materials used.
– Container Closure System: Description and discussion of the container closure system.
– Stability: Stability summary and conclusions, post-approval stability protocol and stability commitment, and stability data.
For the Drug Product (3.2.P):
– Description and Composition: Information on the drug product’s description and composition.
– Pharmaceutical Development: Components of the drug product, drug product, manufacturing process development, container closure system, microbiological attributes, and compatibility.
– Manufacture: Information on manufacturers, batch formula, description of manufacturing process and process controls, controls of critical steps and intermediates, and process validation and/or evaluation.
– Control of Excipients: Specifications, analytical procedures, validation of analytical procedures, and justification of specifications.
– Control of Drug Product: Similar to the control of excipients, with additional focus on the drug product itself.
– Reference Standards or Materials: Information on the reference standards or materials used.
– Container Closure System: Description and discussion of the container closure system.
– Stability: Studies undertaken, results and conclusions, proposed storage conditions, and shelf life. Each section within Module 3 is meant to provide detailed information that supports the quality and safety of the pharmaceutical product. The information provided in Module 3 forms the basis for the Quality Overall Summary in Module 2, which summarizes and cross-references the detailed information found in Module 3


very useful information